
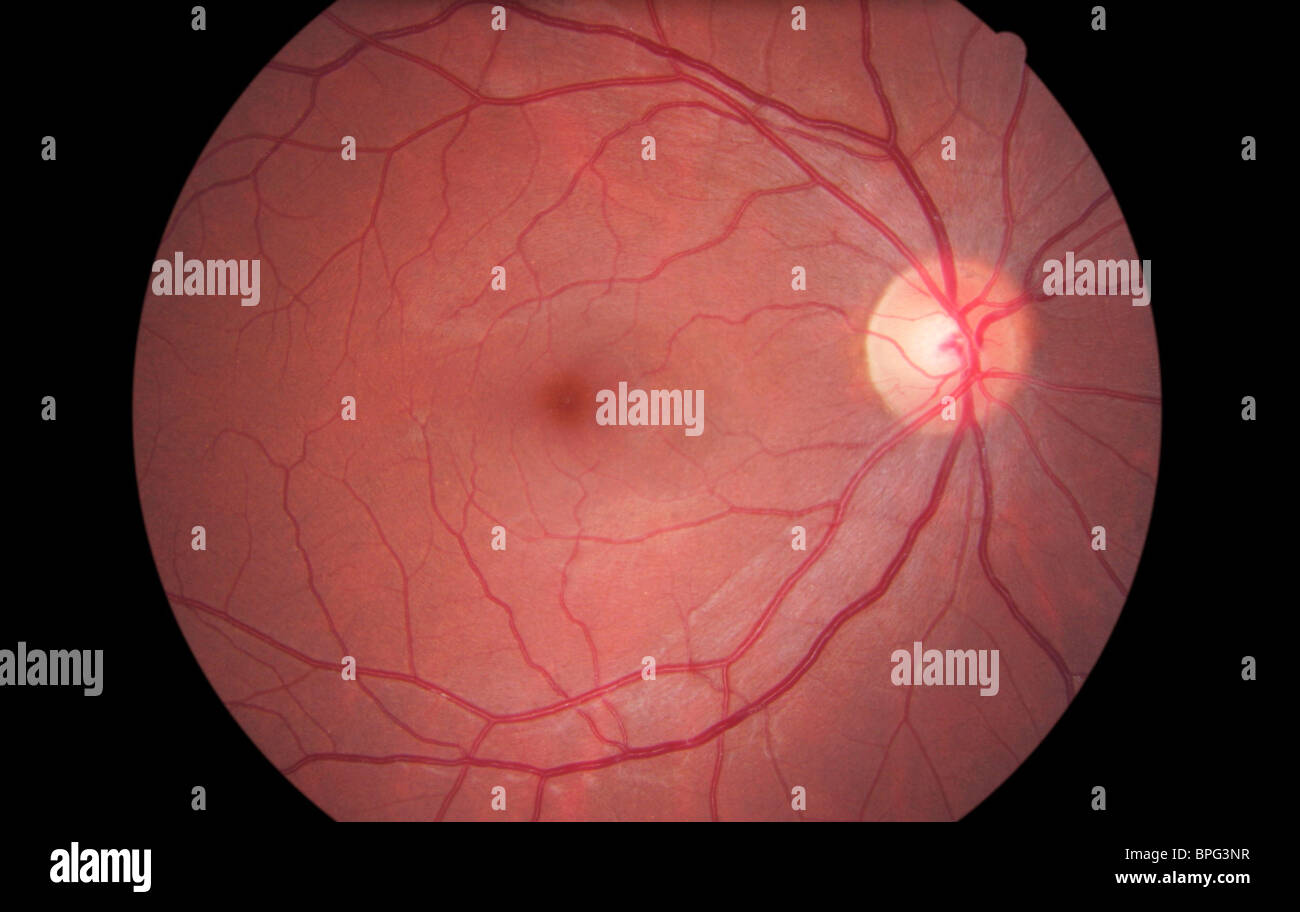

8 GFP is excitable at the wavelength of the autofluorescence mode (488 nm) of a standard cSLO device therefore, we were able to visualize smooth muscle cells and contractile pericytes within the vessel walls of retinal arterioles and venules. Because capillary pericytes do not feature contractile properties, only the precapillary and postcapillary vessels are stained, and the expression pattern as such mirrors the autoregulative/contractile capacity of the retinal vasculature (Tsai JY, et al. Only cells with intrinsic expression of smooth muscle type α-actin also express GFP, resulting in specific GFP labeling of the vessel wall by the fluorescence of smooth muscle cells and a subgroup of pericytes with smooth muscle type α-actin expression. To make the outer diameter of the retinal vessels accessible in vivo, we used a transgenic animal model that expresses green fluorescent protein (GFP) under the transcriptional control of the smooth muscle type α-actin promoter (αSMA). 7 In conclusion, AVR based on funduscopic evaluation closely reflects the relationship between the arterial and the venous inner diameter and does not include the outer diameter (i.e., the wall) of the vessel. Although these arteries have a strong muscular wall, they appear smaller than the veins, suggesting that the main site of reflection is close to the bloodstream and not near the outside of the vessel. 6 Retinal arteries have a more silvery look, indicating that light is reflected before it reaches the blood itself. 4, 5 In the thin-walled veins, the strong absorbance of green light by hemoglobin leads to the characteristic dark appearance. Assessment of this arteriovenous ratio (AVR ), the ratio of arteriolar diameter to venule diameter, and the general appearance of retinal vessels are based on the normal light reflex of the retinal vasculature, which is formed by the reflection from the interface between the blood column and the vessel wall. In funduscopy, diameters of first- and second-order retinal arterioles and venules approximate a 2:3 ratio.

Because vessel walls are primary targets in common hypertensive and metabolic diseases, αSMA-GFP transgenic mice may prove valuable in the detailed assessment of such disorders in vivo. Although arterioles and venules differ in lumen and vessel wall width, they share a common outer diameter, leading to an AVR od close to unity. Transgenic αSMA-GFP expression in murine vessel wall components allowed quantification of retinal vessel outer diameters in vivo. The mean AVR based on either inner diameter (AVR id = 0.72 ± 0.08) or outer diameter (AVR od = 0.97 ± 0.09) measurements were significantly different ( P < 0.01). In αSMA-GFP mice, autofluorescence imaging of the GFP-marked vascular walls also allowed the determination of outer vessel diameters. Native cSLO imaging and angiography yielded only inner vessel diameters similar to those observed through clinical funduscopy. Spectral-domain-OCT and ERG were performed to control for integrity of retinal structure and function in vivo and histology to demonstrate the location of GFP expression. Here the authors used a transgenic mouse model to quantify AVR in vivo based on total vessel dimensions (wall and lumen).Ĭonfocal scanning laser ophthalmoscopy (cSLO) and indocyanine green angiography of the retinal vasculature were performed in wild-type and transgenic mice expressing green fluorescent protein (GFP) under the transcriptional control of the smooth muscle type α-actin (αSMA) promoter. Because vascular walls are typically not visible in funduscopy, clinical AVR estimation is based on the lumen rather than the entire vessel diameter. Retinal blood vessel diameter and arteriovenous ratio (AVR) are commonly used diagnostic parameters.


 0 kommentar(er)
0 kommentar(er)
